Background: Allogeneic hematopoietic cell transplantation (HCT) is curative for many high-risk hematological malignancies. However, graft-versus-host disease (GVHD) causes morbidity, mortality, and reduced quality of life after HCT. Removal of all T cells from hematopoietic cell grafts (pan-T cell depletion; pan-TCD) significantly reduces GVHD but is associated with impaired immune reconstitution and increased non-relapse mortality due partly to opportunistic infections. We developed a novel strategy to remove CD45RA + naïve T cells (T N) from peripheral blood stem cell grafts (PBSC) that is associated with a low incidence of serious acute GVHD (aGVHD) and an exceptionally low incidence of chronic GVHD (cGVHD) (Bleakley, JCO 2022). Although CD45RA-depletion removes T N and other CD45RA-expressing effector T cell populations from PBSC along with most NK and B cells, T N-depletion (T ND) does not appear to increase infections or have a major negative impact on immune reconstitution. Understanding subtle differences in lymphocyte population dynamics after T ND compared to T cell-replete (TR) HCT will enable further graft engineering advances and may provide broader insights into human HCT immunobiology.
Objective: To evaluate lymphocyte reconstitution after T ND HCT.
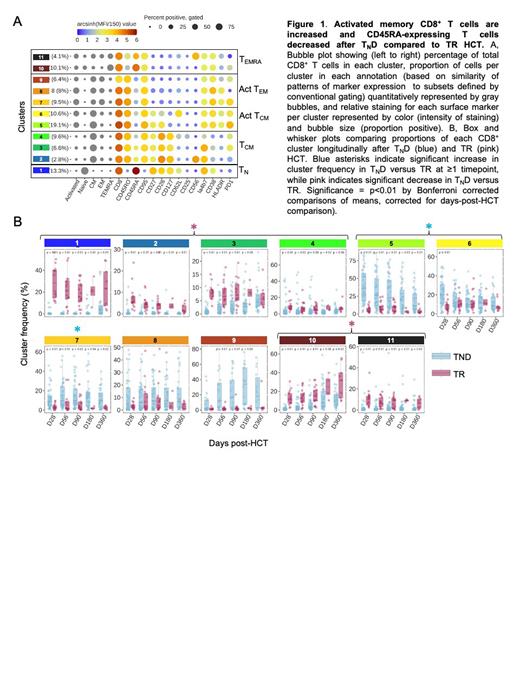
Methods and Results: We compared blood lymphocyte populations in T ND HCT recipients (n=47) to those in TR HCT controls (n=19) at days 28, 56, 90, 180, and 360 post-HCT using multiparametric flow cytometry. To complement standard Boolean analysis, we employed a metaclustering approach to simultaneously evaluate ~20 cell surface markers for each class of lymphocytes (CD8 + and CD4 + T cells, NK cells, and B cells) over scores of batches of recipient and control samples. Absolute numbers of total CD8 + T cells were similar in T ND compared to TR at all timepoints, whereas total CD4 + T cell numbers were consistently lower in T ND until 1 year post-HCT. Using conventional gating and high-dimensional clustering analyses, we identified several key differences in T cells after T ND. CD45RA-expressing populations (T N and effector memory re-expressing CD45RA [T EMRA]) in CD8 + and CD4 + T cells were rare in CD45RA-depleted PBSC and remained decreased in T ND HCT recipients until around 1 year (CD8 +, Fig. 1). Activated CD8 + and CD4 + central and effector memory (T CM, T EM) T cells were increased proportionally and in absolute numbers in T ND early post-HCT (CD8 +, Fig. 1). Absolute numbers of regulatory T cells (Treg) were decreased in T ND; consequently, ratios of activated CD8 + and CD4 + to Treg were both markedly increased early post-HCT, despite a similar incidence of aGVHD in T ND and TR recipients. Although most NK cell populations were removed from CD45RA-depleted PBSC, absolute numbers of NK cells in all compartments, including both activated and inhibited, were increased in T ND recipients early post-HCT compared to TR. Like NK cells, most B cells except for plasmablasts and plasma cells were removed by CD45RA-depletion of PBSC. Immature, naïve, and memory B cells were reduced very early post-T ND HCT, but substantial B cell recovery occurred within the first 2-3 months.
Conclusions: We present the first detailed description of lymphocyte reconstitution after T ND HCT. Despite the major effect CD45RA-depletion has on graft composition-removing all T N and T EMRA from the T cell compartment and most NK and B cells-NK and B cell recovery occurred early, and by 1 year post-HCT there was little difference in lymphocyte populations between T ND and TR. This is consistent with hematopoietic progenitors, not mature lymphocytes, as the source of B and NK cell reconstitution. Among T cells, the most striking findings were an increase in activated T CM and T EM CD4 + and CD8 + T cells early after T ND HCT, and increased ratios of activated T cells to Treg. Although elevated blood T effector/Treg ratios have been associated with cGVHD, cGVHD was uncommon and steroid-responsive after T ND HCT. Whether increased activated T cells reflect a reduction in Treg-mediated suppression after HCT, bystander T cell activation, activation by antigen (e.g., alloantigen, latent herpes viruses, microbiota), or multiple factors are questions we are now investigating. We are also exploring associations between lymphocyte populations early post-T ND HCT and clinical outcomes; these could serve as biomarkers to facilitate early intervention to prevent or treat GVHD, relapse, or infection.
Disclosures
Shlomchik:BlueSphere Bio: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Orca Bio: Consultancy, Current holder of stock options in a privately-held company. Newell:Immunoscape: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Neogene Therapuetics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Nanostring Technologies: Membership on an entity's Board of Directors or advisory committees. Bleakley:Orca Bio: Consultancy; Miltenyi Biotec: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal